Abstract

Introduction.
Patients with Myelofibrosis (MF) are considered fragile and thus eligible in Italy for COVID-19 BNT162b2 mRNA vaccination. According to the International Prognostic Scoring System (IPSS), patients with intermediate and high MF, may receive clinical benefits from ruxolitinib, the first approved JAK1/JAK2 inhibitor. Given the potent anti-inflammatory properties of ruxolitinib against immunocompetent cells, we previously reported a lower but non-statistically absolute IgG anti-Spike humoral response in vaccinated MF patients treated with ruxolitinib. In the present report we extended the cohort of MF patients.
Methods. All MF patients received 2 injections of 30 ug per dose of BNT162b2 mRNA COVID-19 vaccine 3 weeks apart, according to the standard protocol. After injection, mild pain at the injection site was frequently reported. No serious adverse events were registered. The serum level of IgG anti-Spike glycoprotein was tested after a median time of 45 days (range 40-60) from the second vaccine dose, using the approved anti-SARS-CoV-2 IgG CLIA (LIAISON® SARS-CoV-2 TrimericS IgG assay, Diasorin, Saluggia, Italy). An Arbitrary Units per milliliter (AU/mL) ratio of <12.0 was considered to be negative, 12.0-15.0 AU/mL to be borderline and >15 AU/mL to be positive. A conversion of AU/mL to binding antibody units (BAU/mL) as recommended by the World Health Organization (WHO) guidelines was achieved considering the following equation: BAU/mL = 2.6*AU/mL.
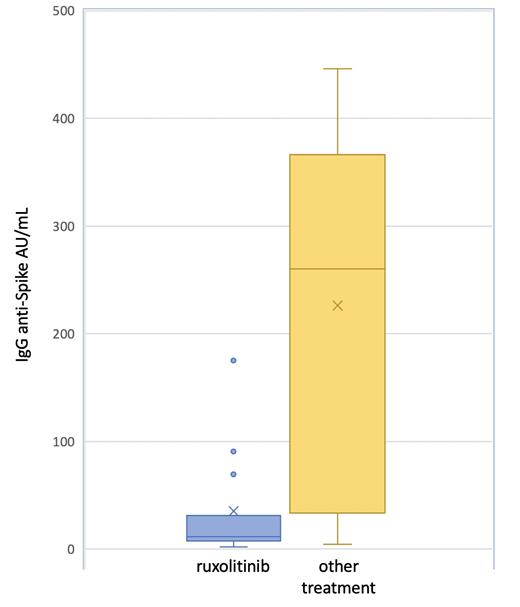
Results. Overall, 30 MF patients (median age 65 years, range 48-83) were vaccinated. A diagnosis of primary MF was reported in 21 cases (70%), post essential thrombocythemia-MF in 6 (20%) patients and post polycythemia vera-MF in 3 (10%) patients; 23 out of 30 patients (76.6%) were positive for the JAK2V617F, 5 (16.6%) for CALR mutation, 1 (3.3%) for MPL mutation and 1 patient (3.3%) resulted triple negative. Splenomegaly was observed in 14 patients (46%) and 19 (63.3%) reported comorbidities. Nineteen patients (63.3%) were classified as DIPSS low or intermediate-1 risk, and 11 (36.6%) as intermediate-2 or high risk. Fifteen patients (50%) were receiving ruxolitinib, at a median total dose of 20 mg/die (range 20-40 mg) and the remaining 15 patients other treatments (8 patients hydroxyurea and 7 only supportive therapy). None of the patients reported COVID-19 infection neither previous nor subsequently to vaccination. Overall, a positive immune response against COVID-19 was observed in 8 out of 15 patients (53.3%) in the ruxolitinib group, in comparison with 13 out 15 patients (86.6%) in the other treatment group (p=0,046). The absolute IgG anti-Spike value was lower in the ruxolitinib group (median 35.2±49.81) in comparison with the other group (median 226.1±163.9; p=<0.001), Figure 1. In univariate analysis, only ruxolitinib treatment was found associated with a lower humoral immune response to the vaccine.
Conclusions. MF patients under ruxolitinib achieved a lower humoral immune response in comparison with MF patients who underwent other treatments. No COVID-19 infection was observed in both groups after vaccination, after a median follow up of 3 months since the second dose. Whether patients with a potential insufficient humoral response to vaccine will benefit from a third dose of BNT162b2 mRNA COVID-19 vaccine is a matter of further investigation. Our preliminary data need to be confirmed in larger cohort of MF patients.
Murru: Abbvie: Consultancy, Honoraria, Other: travel and accommodation; Janssen: Consultancy, Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal